Getting ready for a high-altitude adventure? If so, knowing how elevation changes impact boiling times and temperatures for H2O can be the difference between a successful trip and a disaster.
At higher elevations boiling H2O isn’t the same as boiling it at sea level, and getting to grips with the difference between the two requires taking a quick dip into science and physics. Sound complicated? It isn’t. With a few pointers, you’ll have all the know-how you need to cook, prepare safe drinking water, and make that all-important morning brew anywhere!
In this blog post, we’ll explore the necessary adjustments you need to make when cooking, baking, or boiling at varying elevations. Whether you plan on taking on a big mountain or are camping out a few thousand feet above sea level, we have you covered!
Table of Contents
How to Boil Water At Higher Elevation: all you need to know
First Things First: What Are ‘High Altitudes’
While this varies depending on who you ask, the most commonly cited elevation is around 3,000 feet in elevation.
This may seem low to many hikers and mountaineers, granted. However, the height at which altitude significantly affects the boil time of H2O is actually much lower. How much lower? Read on!

Boiling Water at Higher Elevations Vs Sea Level
The decrease in air pressure (aka atmospheric pressure) at altitude impacts various things, including the weather, our respiratory and circulatory systems, and the boiling point of water.
With regard to boiling water, what interests us campers, hikers, and mountaineers is the point at which H2O molecules transform from the liquid phase to a vapor (i.e. boils) when heated. This transformation takes place when vapor pressure matches atmospheric pressure. Because atmospheric pressure decreases the higher you go, water boils at correspondingly lower temperatures.

Let’s have a look at some examples and figures to demonstrate the point.
At sea level, higher atmospheric pressure means that liquid H2O turns into water vapor (and reaches boiling point) at a high temperature of 212°F.
At 3,000 feet above sea level, however, a slightly lower atmospheric pressure is observed, and H2O boils at 208°F. At 5,000 feet, it’s lower still, and the boiling point is 203°F. If you’re in Denver (5,279ft), it’s lower still and will boil at 202°F. At 10,000 feet, it’s lower still and will boil at 194°F. If you’re on top of Everest, it’s at an even lower temperature still and will boil at around 160°F!
Let’s reiterate that main takeaway: as atmospheric pressure decreases with increasing altitude, the boiling point of H2O decreases. Higher pressure equals a higher boiling temperature. When altitude increases by 500 feet, the boiling point of water drops by a fraction less than 1°F.
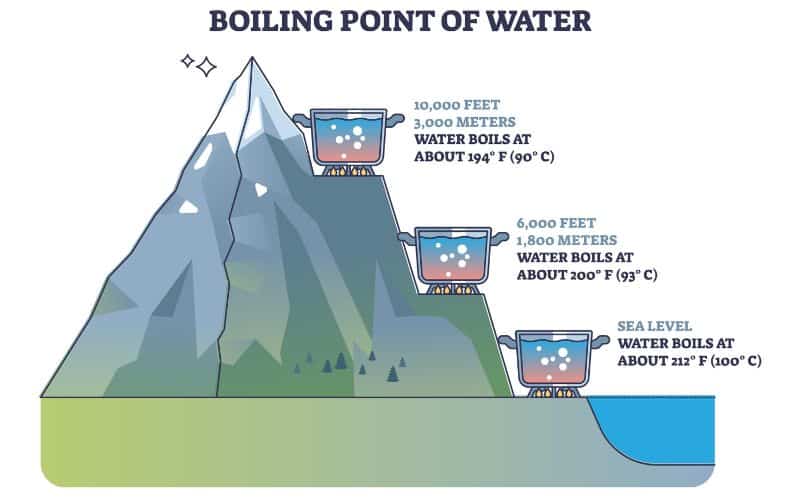
So, what does this mean? The first point to note is that, contrary to what many think, boiling H2O at altitude is quicker than at lower elevations. While colder temperatures and strong winds may mean it takes longer to heat water from one temperature to another, in the same conditions, the fact remains: the higher you are, you’ll see water boil faster
The second point of note concerns how this impacts cooking and water purification when you’re camping or hiking at high altitude, which we’ll deal with below.
What This Means for Cooking and Drinking Water
Above, we demonstrated how H2O has a lower boiling point at higher elevations. If you haven’t put two and two together yet, let us get to the point now: a lower boiling point means less heat is produced, which means longer cooking times.
At 3,000 feet, H2O boils at around 4 degrees cooler than at sea level. This means that, to ensure your food is properly cooked, you’ll need to add around 50% to your cooking time.
At Denver’s elevation (just over 5,000 feet), you’ll need to double your cooking time. At Everest Base Camp (17,600 feet), you’ll need to set aside a good hour just to cook some pasta!

Why is this important? Two reasons:
First of all, eating uncooked meats and poultry, of course, is dangerous. If you’re cooking these in boiled H2O, therefore, avoiding food poisoning means you’ll need to bear all of the above in mind.
Secondly, boiling is the most effective means of purifying water, but only if you do it right. Given all that we’ve covered above, doing it right is a little trickier up high than at sea level.
At sea level, you can purify H2O, eliminating 99.999999% of protozoa, bacteria, and viruses, by leaving it to boil for just one minute. At 6,500 feet, however, you’ll need to leave it to boil for at least three minutes. Above 10,000 feet, it’s safest to leave it to boil for at least 5 minutes.

Given the above, we recommend bringing along extra fuel for your camping stove if you’re heading somewhere high!
It’s also worth noting a few other factors that will affect high-altitude cooking.
First up, your H2O will evaporate quicker, meaning you’ll need slightly more of it when preparing certain dishes or filling your pot to make that morning cup of coffee (which may be an espresso rather than the americano you’d hoped for by the time you lift the lid!).
Secondly, leavening agents like yeast, baking soda, baking powder, whipped egg whites, and cream all expand more at high elevations, so bring along a larger dish for those breakfast pancakes or that camp banana bread!

Boiling Water at Higher Altitudes…
If you’re heading somewhere high, keeping the above info in mind and adjusting your cooking time accordingly will ensure you stay healthy and well-hydrated throughout your trip!
If you have any questions or comments about boiling water at higher altitudes, drop us a line in the box below. And if you’d like to share this post with your friends, please do!

